09
Dec
National Institute of Biology (NIB) is coordinating 7. Framework programme project which has 3,3 million EUR budget.
National Institute of Biology (NIB) is coordinating 7. Framework programme project which has 3,3 million EUR budget.
Project CytoThreat (Fate and effects of cytostatic pharmaceuticals in the environment and the identification of biomarkers for and improved risk assessment on environmental exposure)
In October 2010 European Commission and National Institute of Biology signed a research contract about implementation of Collaborative project CytoThreat. Project proposal was prepared in January 2010 by NIB and nine European partners within FP7 programme Environment (FP7-ENV-2010). After positive evaluation of project proposal and negotiation that concluded in autumn 2010, European Commission will co-financed project in the amount of 2.582.959 EUR. Project duration is four years; from 1.1.2011 till 31.12.2014. So far NIB participated in numerous FP projects, but it is the first time that is coordinating a FP project. Project coordinator is prof. dr. Metka Filipič. Project homepage will be prepared within the first months after the project commencement.
Abstract of the Project:
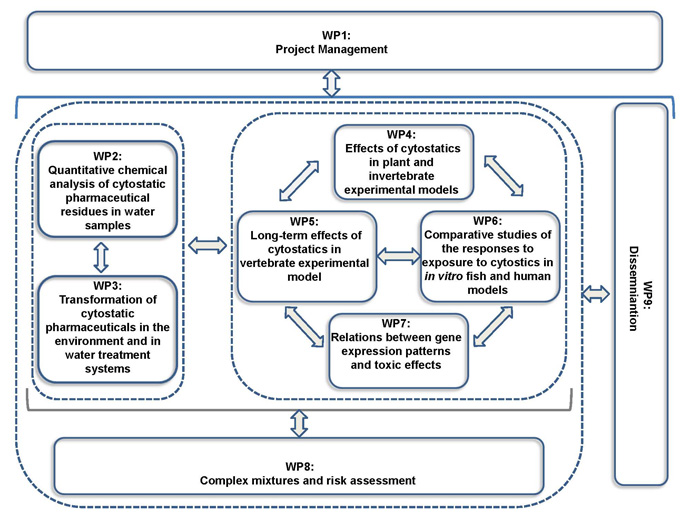
CytoThreat addresses the need to assess the risks of pharmaceuticals released in the environment, focusing on cytostatic drugs because they are highly hazardous compounds due to their genotoxic properties which may cause unexpected long term effects. Their release in the environment may lead to systemic ecological effects and increased cancer incidence, reduced fertility and malformations in the offspring in humans. The occurrence, distribution and fate of selected widely used cytostatics in different aquatic matrices, their acute and chronic toxicity and impact on the stability of the genetic material in a variety of aquatic organisms representing different trophic levels is addressed to provide data sets necessary for scientifically based risk assessment. Special emphasis is put on the combined effects of environmentally relevant mixtures. A combination of state-of-the art analytical chemistry, in vivo and in vitro systems, and 'OMICS' technologies is applied. In vivo studies with zebrafish models 131m at identifying linkages between the genomic profiles, exposure t.ond1tions and adverse effects in vertebrates to identify molecular biomarkers 'or adverse effects of specific groups of cytostatics to be used as diagnostic markers and for predicting synergistic effects of combined exposures. Comparative in vitro genotoxicity and tral1scriptomic studies with zebrafish and human derived cells will provide additional information for the extrapolation of toxicological data to humans. Comparisons with the hazardous effects of other groups of pharmaceuticals will provide knowledge on the magnitude of the problem. CytoThreat will generate new knowledge on environmental and health risks of cytostatics and provide objective arguments for recommendations and regulations. Partners form 5 member states and 2 associated countries with complementary expertise in analytical chemistry, aquatic and genetic toxicology, and genomics and bioinformatics are involved.
Partners:
• National Institute of Biology - coordinator
• The Jozef Stefan Institute (JSI), Slovenia
• RR&Co d.o.o., Slovenia
• Medical University of Vienna (MUW),, Austria
• Szent István University (SZIE), Hungry,
• The Second University of Naples (SUN) Italy,
• Spanish Council for Scientific Research (CSIC), Spain
• The Institute for Medical Research and Occupational Health (IMI), Croatia
• Institute for Multidisciplinary Research (IMSI), Srbia