Research work on grapevine yellows phytoplasma
Research on phytoplasma at the Department of Biotechnology and Systems Biology started in the 1990s. At the beginning efforts were focused into setting up molecular biology detection techniques for efficient control over the phytosatinary status of grapevine yellows, a disease caused by phytoplasma on grapevine. Research on phytoplasma at the Department of Biotechnology and Systems Biology started in the 1990s. At the beginning efforts were focused into setting up molecular biology detection techniques for efficient control over the phytosatinary status of grapevine yellows, a disease caused by phytoplasma on grapevine (Seljak et al., 2000; Petrovič et al., 2004). Later the methods for detection of fruit tree phytoplasma were established. At the beginning the detection systems were based on classical PCR reactions (polymerase chain reactions) and RFLP (restriction fragment length polymorphism) and were later substituted with a more advanced technique - real-time PCR. Combined efforts with Phytosanitary Administration of the Republic of Slovenia and phytosanitary inspectors resulted in setting-up a network for screening of grapevine yellows in vineyards throughout the country. It became clear very soon that the control over spread of phytoplasma infections can only be achieved through the understanding of these pathogenic organisms and their interaction with their plant hosts. This was the start of research on phytplasma-grapevine interaction at the Department of Biotechnology and Systems Biology at National Institute of Biology.
Phytoplasma
Phytoplasma are cell-wall-less bacterial pathogens belonging to class Mollicutes and cause over 1000 different plant diseases throughout the world. In plants they live in nutrient rich phloem. The colonisation of phytoplasmas in the infected plant depends on the season, plant organ, plant host and the pathogen itself. Therefore phytoplasma cause a range of symptoms that depend on their interference with the plant host physiology. Phytoplasmas are transmitted with insects from the class Hemiptera that feed on the phloem sap. Major genomic changes occurred in the evolution of phytoplasmas (they have a very small genome) which might have caused their dependence on plant and insect hosts. Up to now attempts to culture phytoplasma in the absence of plants or insects have failed therefore their physiology and genetic s are poorly understood.
The most widespread grapevine related phytoplasma in Europe and the world are phytoplasmas that causes ‘bois noir’ disease and phytoplasmas that causes ‘flavescence dorée’. A common term for all the diseases caused by phytoplasma on grapevine is Grapevine yellows. ‘Bois noir’ phytoplasma is spread throughout the world and is also present in all winegrowing regions in Slovenia. Typical symptoms on the infected plants include yellowing (redding in red varieties) and downward curling of leaf laminas, uneven lignifications of canes, flower abortion and berry withering (Figures 1-3). Consequently the yield is reduced.



Research work on phytoplasma related grapevine diseases
First basic research of phytoplasma pathogenesis at the Department goes back into late 1990s when the interaction between phytoplasma from the group 16SrI-B and maize was studied.
Later on we selected grapevine cultivar Chardonnay infected with ‘bois noir’ phytoplasma (group 16SrXII) as the model for our research work. We used state-of-the-art gene expression techniques for the study of this interaction with the aim of understanding the processes in the grapevine-infected tissue. Because we wanted to obtain biologically relevant data we did not want to use controlled environmental laboratory conditions but used a production vineyard in a small village Vipolže in Goriška Brda as our experimental field. Vast majority of experiments in science are produced in controlled laboratory conditions because unpredictable environmental changes and effects can hide the changes we are looking for. However, we were willing to take the challenge. We received a very worm welcome and an understanding partner in the biggest wine cellar in that region “Vinska klet Goriška Brda, Dobrovo” who made our research work in a vineyard of one of their cooperates possible and thus saved us time and energy (Figure 4). Our sampling was focused on central leaf veins of a few leaves per shoot so that we did not leave much damage on the plants or grape berries (Figure 5).


The first step to research of phytoplasmas is their accurate detection in the samples. Conventional PCR tests proved not to be sensitive enough, required large amounts of sample material and were laborious and time consuming. Therefore we developed a new real-time PCR based detection method for grapevine yellows that was more sensitive and much faster (Hren et al., 2007). The method was compared with the conventional PCR tests on samples from the whole sampling season before being introduced into the laboratory for routine testing. Along with the complete detection system the new real-time PCR based method gradually replaced conventional PCR tests. Currently it is the main detection technique used for grapevine yellows phytoplasma in Slovenia.
DNA microarray technology was used to profile gene expression of grapevine plant response to phytoplasma infection. We were able to follow expression of 14.500 grapevine genes in central leaf veins in a sample that weight approximately one tenth of a gram. This technology was introduced to our Department through the collaboration with INRA (L'Institut national de la recherche agronomique) from Montpellier, France.
Experimental design was set-up (Figure 6) where the symptomatic (infected) und non-symptomatic (healthy) plants were screened for gene expression of three marker genes with real-time PCR (Hren et al., 2009a), followed by DNA-microarray analysis of the same samples based on the results from marker gene analysis (Figure 7).
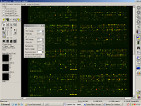
Figure 7: A step from DNA-microarray analysis (image analysis - data extraction from image).
Immense quantity of data was obtained from DNA-microarray experiments (table with 14.500 rows and tens of columns). They had to be transformed into a form that was more comprehendible and less confusing for the human eye and mind. For this purpose we contacted a German bioinformatic group from Max Planck Institute in Golm (Germany) and with common efforts adapted their existing visualisation software MapMan (at that time developed only for plant Arabidopsis thaliana) for grapevine (Vitis vinifera). This large bioinformatic effort was paid-off with a publication in BMC Plant Biology, where the paper is already marked “highly accessed” (Rotter et al., 2009; Figure 8).

DNA-microarrays enables monitoring of a large number of genes simultaneously, however it lacks the sensitivity and dynamic range of real time-PCR. Therefore it is vital that DNA-microarray results are validated independently with real-time PCR. For this purpose we selected several genes from different biological processes. Results matched perfectly, reassuring us that we could interpret the microarray results with high confidence. Based on gene expression data we built a model of carbohydrate metabolism in leaf tissues of phytoplasma infected grapevine plants (Figure 9). Gene expression analyses with real-time PCR in field collected samples can also be used to find marker genes for the infection even before the appearance of symptoms.
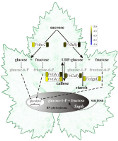
Figure 9: We assume that 'bois noir' phytoplasma has a complete gene for sucrose phosphorylase, an enzyme important for sucrose cleavage into glucose-1-phospahte and fructose. How the enzyme degradation products might be further utilized by phytoplasmas is currently not known, therefore we propose this model. Gene expression is colour-coded (yellow colour means that the gene was more expressed in infected plants and blue means that the gene was expressed more in healthy plants). VvvInv2 encodes vacuolar acid invertase 2, VvSuSy encodes sucrose synthase, VvGlc1 and VvGlc2 encode two 1.3-b-glucanases, VvCaSy encodes callose synthase, VvAgpL encodes large subunit of ADP-glucose pyrophosphorylase and Suph encodes phytoplasmic sucrose phosphorylase.
The research work was recently published in GMC Genomics (Hren et al., 2009b). This is the second paper in the world using DNA-microarrays to investigate gene expression changes in connection to grapevine yellows. All publications related to our work on grapevine yellows represent a big success and a step forward for our Department into the international research field of this important disease using the most advanced molecular biology techniques. This is a proof that even a small research group can contribute to the advances of a certain field of biology, especially by integrating different approaches, such as laboratory and bioinformatics.
And the future?
We will continue the research grapevine yellows and will complement the work with analyses of proteins, enzyme activity and plant hormones in grapevine. We will also use new bioinformatic approaches such as promoter analysis in order to shed light onto the regulation of gene expression in infected plants. In collaboration with a research group from University of Udine (Italy), we will extend our approach to study the phenomenon known as ‘recovery’ (a spontaneous development of resistance/tolerance in certain phytoplasma infected grapevine plants).
In the end we would like to present the team who worked on grapevine yellows phytoplasmas from the early years up to now: Nataša Petrovič, Jana Boben, Jernej Brzin, Maja Ravnikar, Kristina Gruden, Matjaž Hren, Petra Nikolič, Marina Dermastia, in Ana Rotter.
Collaborations
Publications
HREN, Matjaž, BOBEN, Jana, ROTTER, Ana, KRALJ NOVAK, Petra, GRUDEN, Kristina, RAVNIKAR, Maja. Real-time PCR detection systems for Flavescence dorée and Bois noir phytoplasmas in grapevine : comparision with conventional PCR detection and application in diagnostics. Plant Pathol., 2007, vol. 56, str. 785-796. [COBISS.SI-ID 1773135]
HREN, Matjaž, RAVNIKAR, Maja, BRZIN, Jernej, ERMACORA, Paolo, CARRARO, Luigi, BIANCO, P.A., CASATI, P., BORGO, M., ANGELINI, E., ROTTER, Ana, GRUDEN, Kristina. Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathol., 2009a, vol. 58, str. 170-180. http://dx.doi.org/10.1111/j.1365-3059.2008.01904.x, doi: doi:10.1111/j.1365-3059.2008.01904.x. [COBISS.SI-ID 1888335]
HREN, Matjaž, NIKOLIĆ, Petra, ROTTER, Ana, BLEJEC, Andrej, TERRIER, Nancy, RAVNIKAR, Maja, DERMASTIA, Marina, GRUDEN, Kristina. 'Bois noir' phytoplasma induces significant reprogramming of the leaf transcriptome in the field grown grapevine. BMC Genomics, 2009b, vol. 10, no. 460, 38 str. http://www.biomedcentral.com/1471-2164/10/460, doi: 10.1186/1471-2164-10-460. [COBISS.SI-ID 2103375]
PETROVIČ, Nataša, BOBEN, Jana, RAVNIKAR, Maja. Laboratorijsko testiranje trsnih rumenic v Sloveniji kaže na splošno prisotnost rumenice tipa počrnelosti lesa = Laboratory testing of grapevine yellows in Slovenia indicates a widespread presence of Bois noir. Acta agric. Slov.. [Tiskana izd.], 2004, vol. 83, št. 2, str. 313-322. [COBISS.SI-ID 4167545]
ROTTER, Ana, HREN, Matjaž, GRUDEN, Kristina. Gene expression profiling in susceptible interaction of grapevine with its fungal pathogen Eutypa lata : extending MapMan ontology for grapevine. BMC plant biol. (Online), 2009, vol. 9, no. 104, str. 1-14. http://dx.doi.org/10.1186/1471-2229-9-104, doi: 10.1186/1471-2229-9-104. [COBISS.SI-ID 2058319]
SELJAK, Gabrijel, PETROVIČ, Nataša. Diffusione e stato della ricerca delle malattie da fitoplasmi in Slovenia = The presence of phytoplasma diseases in Slovenia and the situation of the research. Petria, 2000, letn. 10, št. 2, str. 133-139. [COBISS.SI-ID 12661721]
Apart from the listed scientific papers we presented the research results also in several international and Slovenian conferences in the form of abstracts, posters and oral presentations.
Phytoplasma
Phytoplasma are cell-wall-less bacterial pathogens belonging to class Mollicutes and cause over 1000 different plant diseases throughout the world. In plants they live in nutrient rich phloem. The colonisation of phytoplasmas in the infected plant depends on the season, plant organ, plant host and the pathogen itself. Therefore phytoplasma cause a range of symptoms that depend on their interference with the plant host physiology. Phytoplasmas are transmitted with insects from the class Hemiptera that feed on the phloem sap. Major genomic changes occurred in the evolution of phytoplasmas (they have a very small genome) which might have caused their dependence on plant and insect hosts. Up to now attempts to culture phytoplasma in the absence of plants or insects have failed therefore their physiology and genetic s are poorly understood.The most widespread grapevine related phytoplasma in Europe and the world are phytoplasmas that causes ‘bois noir’ disease and phytoplasmas that causes ‘flavescence dorée’. A common term for all the diseases caused by phytoplasma on grapevine is Grapevine yellows. ‘Bois noir’ phytoplasma is spread throughout the world and is also present in all winegrowing regions in Slovenia. Typical symptoms on the infected plants include yellowing (redding in red varieties) and downward curling of leaf laminas, uneven lignifications of canes, flower abortion and berry withering (Figures 1-3). Consequently the yield is reduced.

Figure 1: Grapevine yellows symptoms (yellowing, downward curling of leaf laminas).

Figure 2: Grapevine yellows symptoms (yellowing, downward curling of leaf laminas).

Figure 3: Grapevine yellows symptoms (berry withering).
Research work on phytoplasma related grapevine diseases
First basic research of phytoplasma pathogenesis at the Department goes back into late 1990s when the interaction between phytoplasma from the group 16SrI-B and maize was studied.Later on we selected grapevine cultivar Chardonnay infected with ‘bois noir’ phytoplasma (group 16SrXII) as the model for our research work. We used state-of-the-art gene expression techniques for the study of this interaction with the aim of understanding the processes in the grapevine-infected tissue. Because we wanted to obtain biologically relevant data we did not want to use controlled environmental laboratory conditions but used a production vineyard in a small village Vipolže in Goriška Brda as our experimental field. Vast majority of experiments in science are produced in controlled laboratory conditions because unpredictable environmental changes and effects can hide the changes we are looking for. However, we were willing to take the challenge. We received a very worm welcome and an understanding partner in the biggest wine cellar in that region “Vinska klet Goriška Brda, Dobrovo” who made our research work in a vineyard of one of their cooperates possible and thus saved us time and energy (Figure 4). Our sampling was focused on central leaf veins of a few leaves per shoot so that we did not leave much damage on the plants or grape berries (Figure 5).

Figure 4: We monitored the same plants in the vineyard from 2004. For easier work we tagged the individual plants.

Figure 5: Preparing the samples in the field (cutting the leaf veins). As soon as the leaf veins are cut they are stored in liquid nitrogen.
The first step to research of phytoplasmas is their accurate detection in the samples. Conventional PCR tests proved not to be sensitive enough, required large amounts of sample material and were laborious and time consuming. Therefore we developed a new real-time PCR based detection method for grapevine yellows that was more sensitive and much faster (Hren et al., 2007). The method was compared with the conventional PCR tests on samples from the whole sampling season before being introduced into the laboratory for routine testing. Along with the complete detection system the new real-time PCR based method gradually replaced conventional PCR tests. Currently it is the main detection technique used for grapevine yellows phytoplasma in Slovenia.
DNA microarray technology was used to profile gene expression of grapevine plant response to phytoplasma infection. We were able to follow expression of 14.500 grapevine genes in central leaf veins in a sample that weight approximately one tenth of a gram. This technology was introduced to our Department through the collaboration with INRA (L'Institut national de la recherche agronomique) from Montpellier, France.
Experimental design was set-up (Figure 6) where the symptomatic (infected) und non-symptomatic (healthy) plants were screened for gene expression of three marker genes with real-time PCR (Hren et al., 2009a), followed by DNA-microarray analysis of the same samples based on the results from marker gene analysis (Figure 7).

Figure 6: Experimental design of gene expression in grapevine with DNA-microarrays and real-time PCR.

Figure 7: A step from DNA-microarray analysis (image analysis - data extraction from image).
Immense quantity of data was obtained from DNA-microarray experiments (table with 14.500 rows and tens of columns). They had to be transformed into a form that was more comprehendible and less confusing for the human eye and mind. For this purpose we contacted a German bioinformatic group from Max Planck Institute in Golm (Germany) and with common efforts adapted their existing visualisation software MapMan (at that time developed only for plant Arabidopsis thaliana) for grapevine (Vitis vinifera). This large bioinformatic effort was paid-off with a publication in BMC Plant Biology, where the paper is already marked “highly accessed” (Rotter et al., 2009; Figure 8).
Figure 8: MapMan is software which is able to schematically represent metabolic pathways and processes in a plant and is able to visualise the data on expression of specific genes that are involved into a specific process. In this way we get a very fast and detailed overview of what is happening to specific pathways, processes in the plant’s metabolism, e.g. after infection with phytoplasmas. This image represents a scheme of photosynthesis and biotic stress. Each coloured square represents a gene, whose expression is colour-coded (yellow colour means that the gene was more expressed in infected plants and blue means that the gene was expressed more in healthy plants).
DNA-microarrays enables monitoring of a large number of genes simultaneously, however it lacks the sensitivity and dynamic range of real time-PCR. Therefore it is vital that DNA-microarray results are validated independently with real-time PCR. For this purpose we selected several genes from different biological processes. Results matched perfectly, reassuring us that we could interpret the microarray results with high confidence. Based on gene expression data we built a model of carbohydrate metabolism in leaf tissues of phytoplasma infected grapevine plants (Figure 9). Gene expression analyses with real-time PCR in field collected samples can also be used to find marker genes for the infection even before the appearance of symptoms.

Figure 9: We assume that 'bois noir' phytoplasma has a complete gene for sucrose phosphorylase, an enzyme important for sucrose cleavage into glucose-1-phospahte and fructose. How the enzyme degradation products might be further utilized by phytoplasmas is currently not known, therefore we propose this model. Gene expression is colour-coded (yellow colour means that the gene was more expressed in infected plants and blue means that the gene was expressed more in healthy plants). VvvInv2 encodes vacuolar acid invertase 2, VvSuSy encodes sucrose synthase, VvGlc1 and VvGlc2 encode two 1.3-b-glucanases, VvCaSy encodes callose synthase, VvAgpL encodes large subunit of ADP-glucose pyrophosphorylase and Suph encodes phytoplasmic sucrose phosphorylase.
The research work was recently published in GMC Genomics (Hren et al., 2009b). This is the second paper in the world using DNA-microarrays to investigate gene expression changes in connection to grapevine yellows. All publications related to our work on grapevine yellows represent a big success and a step forward for our Department into the international research field of this important disease using the most advanced molecular biology techniques. This is a proof that even a small research group can contribute to the advances of a certain field of biology, especially by integrating different approaches, such as laboratory and bioinformatics.
And the future?
We will continue the research grapevine yellows and will complement the work with analyses of proteins, enzyme activity and plant hormones in grapevine. We will also use new bioinformatic approaches such as promoter analysis in order to shed light onto the regulation of gene expression in infected plants. In collaboration with a research group from University of Udine (Italy), we will extend our approach to study the phenomenon known as ‘recovery’ (a spontaneous development of resistance/tolerance in certain phytoplasma infected grapevine plants).Researchers
In the end we would like to present the team who worked on grapevine yellows phytoplasmas from the early years up to now: Nataša Petrovič, Jana Boben, Jernej Brzin, Maja Ravnikar, Kristina Gruden, Matjaž Hren, Petra Nikolič, Marina Dermastia, in Ana Rotter.
Collaborations
- Biologia e Protezione delle Piante, Universita degli Studi di Udine, Italy
- Unité Mixte de Recherche, Sciences Pour l'Œnologie, INRA, Montpellier, France
- Laboratoire de Physiologie et Biochimie Végétales, Université de Poitiers, Poitiers, France
- Max Planck Institute of Molecular Plant Physiology, Golm, Germany
- Department of Genetics and Molecular Biology, IASMA Research Center, Michele a/Adige, Italy
- Wine cellar »Goriška Brda« z.o.o. Dobrovo, Dobrovo, Slovenia
- Kmetijsko gozdarski zavod Nova Gorica, Nova Gorica, Slovenia
- Phytosanitary Administration of the Republic of Slovenia, Ljubljana, Slovenia
Publications
HREN, Matjaž, BOBEN, Jana, ROTTER, Ana, KRALJ NOVAK, Petra, GRUDEN, Kristina, RAVNIKAR, Maja. Real-time PCR detection systems for Flavescence dorée and Bois noir phytoplasmas in grapevine : comparision with conventional PCR detection and application in diagnostics. Plant Pathol., 2007, vol. 56, str. 785-796. [COBISS.SI-ID 1773135]HREN, Matjaž, RAVNIKAR, Maja, BRZIN, Jernej, ERMACORA, Paolo, CARRARO, Luigi, BIANCO, P.A., CASATI, P., BORGO, M., ANGELINI, E., ROTTER, Ana, GRUDEN, Kristina. Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathol., 2009a, vol. 58, str. 170-180. http://dx.doi.org/10.1111/j.1365-3059.2008.01904.x, doi: doi:10.1111/j.1365-3059.2008.01904.x. [COBISS.SI-ID 1888335]
HREN, Matjaž, NIKOLIĆ, Petra, ROTTER, Ana, BLEJEC, Andrej, TERRIER, Nancy, RAVNIKAR, Maja, DERMASTIA, Marina, GRUDEN, Kristina. 'Bois noir' phytoplasma induces significant reprogramming of the leaf transcriptome in the field grown grapevine. BMC Genomics, 2009b, vol. 10, no. 460, 38 str. http://www.biomedcentral.com/1471-2164/10/460, doi: 10.1186/1471-2164-10-460. [COBISS.SI-ID 2103375]
PETROVIČ, Nataša, BOBEN, Jana, RAVNIKAR, Maja. Laboratorijsko testiranje trsnih rumenic v Sloveniji kaže na splošno prisotnost rumenice tipa počrnelosti lesa = Laboratory testing of grapevine yellows in Slovenia indicates a widespread presence of Bois noir. Acta agric. Slov.. [Tiskana izd.], 2004, vol. 83, št. 2, str. 313-322. [COBISS.SI-ID 4167545]
ROTTER, Ana, HREN, Matjaž, GRUDEN, Kristina. Gene expression profiling in susceptible interaction of grapevine with its fungal pathogen Eutypa lata : extending MapMan ontology for grapevine. BMC plant biol. (Online), 2009, vol. 9, no. 104, str. 1-14. http://dx.doi.org/10.1186/1471-2229-9-104, doi: 10.1186/1471-2229-9-104. [COBISS.SI-ID 2058319]
SELJAK, Gabrijel, PETROVIČ, Nataša. Diffusione e stato della ricerca delle malattie da fitoplasmi in Slovenia = The presence of phytoplasma diseases in Slovenia and the situation of the research. Petria, 2000, letn. 10, št. 2, str. 133-139. [COBISS.SI-ID 12661721]
Apart from the listed scientific papers we presented the research results also in several international and Slovenian conferences in the form of abstracts, posters and oral presentations.